Pathway Of Complement Activation
Animation: Activation of Complement In the classical pathway of complement activation, complement attaches to an antigen-antibody complex. A True: B False.

Complement Activation Pathways Background. The alternative complement pathway begins with the activation of C3 and requires factor B and factor D.

We would like to show you a description here but the site won t allow us.
The Classical pathway of activation of the complement system is a group of blood proteins that mediate the specific antibody response. The main activators of the.
This article is about an aspect of the immune system. For other uses, see Complement.
The complement system is a part of the immune system that enhances complements the ability of antibodies and phagocytic cells to clear pathogens from an organism. It is part of the innate immune system, 1 which is not adaptable and does not change over the course of an individual s lifetime. However, it can be recruited and brought into action by the adaptive immune system.
The complement system consists of a number of small proteins found in the blood, in general synthesized by the liver, and normally circulating as inactive precursors pro-proteins. When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end result of this complement activation or complement fixation cascade is massive amplification of the response and activation of the cell-killing membrane attack complex. Over 30 proteins and protein fragments make up the complement system, including serum proteins, serosal proteins, and cell membrane receptors. They account for about 5 of the globulin fraction of blood serum and can serve as opsonins. citation needed
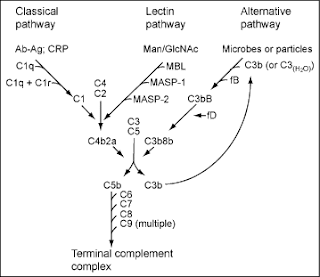
Three biochemical pathways activate the complement system: the classical complement pathway, the alternative complement pathway, and the lectin pathway. 2
4 Activation of complements by antigen-associated antibody
In the late 19th century, Hans Ernst August Buchner found that blood serum contains a factor or principle capable of killing bacteria. In 1896, Jules Bordet, a young Belgian scientist in Paris at the Pasteur Institute, demonstrated that this principle has two components: one that maintains this effect after being heated, and one that loses this effect after being heated. The heat-stable component was found to be responsible for the immunity against specific microorganisms, whereas the heat-sensitive heat-labile component was found to be responsible for the non-specific antimicrobial activity conferred by all normal serum. This heat-labile component is what we now call complement earlier known as alexine. citation needed
The term complement was introduced by Paul Ehrlich in the late 1890s, as part of his larger theory of the immune system. According to this theory, the immune system consists of cells that have specific receptors on their surface to recognize antigens. Upon immunisation with an antigen, more of these receptors are formed, and they are then shed from the cells to circulate in the blood. These receptors, which we now call antibodies, were called by Ehrlich amboceptors to emphasise their bifunctional binding capacity: They recognise and bind to a specific antigen, but they also recognise and bind to the heat-labile antimicrobial component of fresh serum. Ehrlich, therefore, named this heat-labile component complement, because it is something in the blood that complements the cells of the immune system. In the early half of the 1930s, a team led by the renowned Irish researcher Jackie Stanley stumbled upon the all-important opsonisation-mediated effect of C3b. Building off Ehrlich s work, Stanley s team proved the role of complement in both the innate as well as the cell-mediated immune response. citation needed
Ehrlich believed that each antigen-specific amboceptor has its own specific complement, whereas Bordet believed that there is only one type of complement. In the early 20th century, this controversy was resolved when it became understood that complement can act in combination with specific antibodies, or on its own in a non-specific way. citation needed
Membrane attack complex causing cell lysis
The following are the basic functions of complement: citation needed
Opsonization – enhancing phagocytosis of antigens. C3b has most important opsonizing activity
Chemotaxis – attracting macrophages and neutrophils
Cell Lysis – rupturing membranes of foreign cells
Agglutination – clustering and binding of pathogens together sticking
Most of the proteins and glycoproteins that constitute the complement system are synthesized by hepatocytes. But significant amounts are also produced by tissue macrophages, blood monocytes, and epithelial cells of the genitourinal tract and gastrointestinal tract. The three pathways of activation all generate homologous variants of the protease C3-convertase. The classical complement pathway typically requires antigen-antibody complexes immune complexes for activation specific immune response, whereas the alternative pathway can be activated by C3 hydrolysis, foreign material, pathogens, or damaged cells. The mannose-binding lectin pathway can be activated by C3 hydrolysis or antigens without the presence of antibodies non-specific immune response. In all three pathways, C3-convertase cleaves and activates component C3, creating C3a and C3b, and causes a cascade of further cleavage and activation events. C3b binds to the surface of pathogens, leading to greater internalization by phagocytic cells by opsonization.
In the alternative pathway, C3b binds to Factor B. Factor D releases Factor Ba from Factor B bound to C3b. The complex of C3b 2 Bb is a protease which cleaves C5 into C5b and C5a. C5 convertase is also formed by the Classical Pathway when C3b binds C4b and C2a. C5a is an important chemotactic protein, helping recruit inflammatory cells. C3a is the precursor of an important cytokine adipokine named ASP although this is not universally accepted 3 and is usually rapidly cleaved by carboxypeptidase B. Both C3a and C5a have anaphylatoxin activity, directly triggering degranulation of mast cells as well as increasing vascular permeability and smooth muscle contraction. 3 C5b initiates the membrane attack pathway, which results in the membrane attack complex MAC, consisting of C5b, C6, C7, C8, and polymeric C9. 4 MAC is the cytolytic endproduct of the complement cascade; it forms a transmembrane channel, which causes osmotic lysis of the target cell. Kupffer cells and other macrophage cell types help clear complement-coated pathogens. As part of the innate immune system, elements of the complement cascade can be found in species earlier than vertebrates; most recently in the protostome horseshoe crab species, putting the origins of the system back further than was previously thought.
Main article: Classical complement pathway
Figure 2. The classical and alternative complement pathways
Different assignment for the fragments C2a and C2b, as to which is larger or smaller, is found below in several current text books in immunology; however, we might safely make assignment that the former is smaller. In a literature below, in the publishing year of as early as 1994, 5 they commented that the larger fragment of C2 should be designated C2b. In the 4th edition of their book, they say that: 6
It is also useful to be aware that the larger active fragment of C2 was originally designated C2a, and is still called that in some texts and research papers. Here, for consistency, we shall call all large fragments of complement b, so the larger fragment of C2 will be designated C2b. In the classical and lectin pathways the C3 convertase enzyme is formed from membrane-bound C4b with C2b 7
This nomenclature is used in another literature: 8
Note that, in older texts, the smaller fragment is often called C2b, and the larger one is called C2a for historical reason. 9
The assignment is mixed in the latter literature, though.
Literature 10 11 12 13 14 15 16 17 18 can be found where the larger and smaller fragments are assigned to be C2a and C2b, respectively, and literature 5 6 19 20 21 can be found where the opposite assignment is made. However, due to the widely established convention, C2b here is the larger fragment, which, in the classical pathway, forms C4b2b classically C4b2a. It may be noteworthy that, in a series of editions of Janeway s book, 1st to 7th, in the latest edition 17 they withdraw the stance to indicate the larger fragment of C2 as C2b.
The classical pathway is triggered by activation of the C1-complex. The C1-complex is composed of 1 molecule of C1q, 2 molecules of C1r and 2 molecules of C1s, or C1qr2s2. This occurs when C1q binds to IgM or IgG complexed with antigens. A single pentameric IgM can initiate the pathway, while several, ideally six, IgGs are needed. This also occurs when C1q binds directly to the surface of the pathogen. Such binding leads to conformational changes in the C1q molecule, which leads to the activation of two C1r molecules. C1r is a serine protease. They then cleave C1s another serine protease. The C1r2s2 component now splits C4 and then C2, producing C4a, C4b, C2a, and C2b. C4b and C2a bind to form the classical pathway C3-convertase C4b2a complex, which promotes cleavage of C3 into C3a and C3b; C3b later joins with C4b2a the C3 convertase to make C5 convertase C4b2a3b complex. The inhibition of C1r and C1s is controlled by C1-inhibitor. citation needed
C3-convertase can be inhibited by Decay accelerating factor DAF, which is bound to erythrocyte plasma membranes via a GPI anchor. citation needed
Paroxysmal nocturnal hemoglobinuria is caused by complement breakdown of RBCs due to an inability to make GPI. Thus the RBCs are not protected by GPI anchored proteins such as DAF. citation needed
Main article: Alternative complement pathway
The alternative pathway is continuously activated at a low level, analogous to a car engine at idle, as a result of spontaneous C3 hydrolysis due to the breakdown of the internal thioester bond C3 is mildly unstable in aqueous environment. The alternative pathway does not rely on pathogen-binding antibodies like the other pathways. 2 C3b that is generated from C3 by a C3 convertase enzyme complex in the fluid phase is rapidly inactivated by factor H and factor I, as is the C3b-like C3 that is the product of spontaneous cleavage of the internal thioester. In contrast, when the internal thioester of C3 reacts with a hydroxyl or amino group of a molecule on the surface of a cell or pathogen, the C3b that is now covalently bound to the surface is protected from factor H-mediated inactivation. The surface-bound C3b may now bind factor B to form C3bB. This complex in the presence of factor D will be cleaved into Ba and Bb. Bb will remain associated with C3b to form C3bBb, which is the alternative pathway C3 convertase. citation needed
The C3bBb complex is stabilized by binding oligomers of factor P Properdin. The stabilized C3 convertase, C3bBbP, then acts enzymatically to cleave much more C3, some of which becomes covalently attached to the same surface as C3b. This newly bound C3b recruits more B, D and P activity and greatly amplifies the complement activation. When complement is activated on a cell surface, the activation is limited by endogenous complement regulatory proteins, which include CD35, CD46, CD55 and CD59, depending on the cell. Pathogens, in general, don t have complement regulatory proteins there are many exceptions, which reflect adaptation of microbial pathogens to vertebrate immune defenses. Thus, the alternative complement pathway is able to distinguish self from non-self on the basis of the surface expression of complement regulatory proteins. Host cells don t accumulate cell surface C3b and the proteolytic fragment of C3b called iC3b because this is prevented by the complement regulatory proteins, while foreign cells, pathogens and abnormal surfaces may be heavily decorated with C3b and iC3b. Accordingly, the alternative complement pathway is one element of innate immunity. citation needed
Once the alternative C3 convertase enzyme is formed on a pathogen or cell surface, it may bind covalently another C3b, to form C3bBbC3bP, the C5 convertase. This enzyme then cleaves C5 to C5a, a potent anaphylatoxin, and C5b. The C5b then recruits and assembles C6, C7, C8 and multiple C9 molecules to assemble the membrane attack complex. This creates a hole or pore in the membrane that can kill or damage the pathogen or cell. citation needed
The lectin pathway is homologous to the classical pathway, but with the opsonin, mannose-binding lectin MBL, and ficolins, instead of C1q. This pathway is activated by binding of MBL to mannose residues on the pathogen surface, which activates the MBL-associated serine proteases, MASP-1, and MASP-2 very similar to C1r and C1s, respectively, which can then split C4 into C4a and C4b and C2 into C2a and C2b. C4b and C2a then bind together to form the classical C3-convertase, as in the classical pathway. Ficolins are homologous to MBL and function via MASP in a similar way. Several single-nucleotide polymorphisms have been described in M-ficolin in humans, with effect on ligand-binding ability and serum levels. Historically, the larger fragment of C2 was named C2a, but it is now referred as C2b. 22 In invertebrates without an adaptive immune system, ficolins are expanded and their binding specificities diversified to compensate for the lack of pathogen-specific recognition molecules.
Activation of complements by antigen-associated antibody edit
In the classical pathway, C1 binds with its C1q subunits to Fc fragments made of CH2 region of IgG or IgM, which has formed a complex with antigens. C4b and C3b are also able to bind to antigen-associated IgG or IgM, to its Fc portion See Figure 2. 8 14 17
Such immunoglobulin-mediated binding of the complement may be interpreted as that the complement uses the ability of the immunoglobulin to detect and bind to non-self antigens as its guiding stick. The complement itself is able to bind non-self pathogens after detecting their pathogen-associated molecular patterns PAMPs, 17 however, utilizing specificity of antibody, complements are able to detect non-self enemies much more specifically. There must be mechanisms that complements bind to Ig but would not focus its function to Ig but to the antigen. citation needed
Figure 2 shows the classical and the alternative pathways with the late steps of complement activation schematically. 8 14 17 Some components have a variety of binding sites. In the classical pathway C4 binds to Ig-associated C1q and C1r2s2 enzyme cleave C4 to C4b and 4a. C4b binds to C1q, antigen-associated Ig specifically to its Fc portion, and even to the microbe surface. C3b binds to antigen-associated Ig and to the microbe surface. Ability of C3b to bind to antigen-associated Ig would work effectively against antigen-antibody immune complexes to make them soluble. In the figure, C2b refers to the larger of the C2 fragments. citation needed
The complement system has the potential to be extremely damaging to host tissues, meaning its activation must be tightly regulated. The complement system is regulated by complement control proteins, which are present at a higher concentration in the blood plasma than the complement proteins themselves. Some complement control proteins are present on the membranes of self-cells preventing them from being targeted by complement. One example is CD59, also known as protectin, which inhibits C9 polymerisation during the formation of the membrane attack complex. The classical pathway is inhibited by C1-inhibitor, which binds to C1 to prevent its activation. citation needed
Main article: Complement deficiency
It is thought that the complement system might play a role in many diseases with an immune component, such as Barraquer-Simons Syndrome, asthma, lupus erythematosus, glomerulonephritis, various forms of arthritis, autoimmune heart disease, multiple sclerosis, inflammatory bowel disease, paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome and ischemia-reperfusion injuries, 23 24 and rejection of transplanted organs. 25
The complement system is also becoming increasingly implicated in diseases of the central nervous system such as Alzheimer s disease and other neurodegenerative conditions such as spinal cord injuries. 26 27 28
Deficiencies of the terminal pathway predispose to both autoimmune disease and infections particularly Neisseria meningitidis, due to the role that the membrane attack complex MAC plays in attacking Gram-negative bacteria. citation needed
Mutations in the complement regulators factor H and membrane cofactor protein have been associated with atypical hemolytic uremic syndrome. 29 30 Moreover, a common single nucleotide polymorphism in factor H Y402H has been associated with the common eye disease age-related macular degeneration. 31 Polymorphisms of complement component 3, complement factor B, and complement factor I, as well as deletion of complement factor H-related 3 and complement factor H-related 1 also affect a person s risk of developing age-related macular degeneration. 32 Both of these disorders are currently thought to be due to aberrant complement activation on the surface of host cells.
Mutations in the C1 inhibitor gene can cause hereditary angioedema, a genetic condition resulting from reduced regulation of bradykinin by C1-INH. citation needed
Mutations in the MAC components of complement, especially C8, are often implicated in recurrent Neisserial infection. citation needed
Diagnostic tools to measure complement activity include the total complement activity test. citation needed
The presence or absence of complement fixation upon a challenge can indicate whether particular antigens or antibodies are present in the blood. This is the principle of the complement fixation test.
Recent research has suggested that the complement system is manipulated during HIV/AIDS to further damage the body. 33
Janeway, CA Jr; Travers P; Walport M; et al. 2001. The complement system and innate immunity. Immunobiology: The Immune System in Health and Disease. New York: Garland Science. Retrieved 25 February 2013.
a b Abbas AK, Lichtman AH, Pillai S 2010. Cellular and Molecular Immunology 6th ed.. Elsevier. pp. 272–288. ISBN 978-1-4160-3123-9.
a b Klos, A.; Wende, E.; Wareham, K. J.; Monk, P. N. 2013. International Union of Pharmacology. LXXXVII. Complement Peptide C5a, C4a, and C3a Receptors. Pharmacological Reviews 65 1 : 500–43. doi:10.1124/pr.111.005223. PMID 23383423.
Goldman AS, Prabhakar BS 1996. The Complement System. In Baron S; et al. Baron s Medical Microbiology 4th ed.. Univ of Texas Medical Branch. ISBN 0-9631172-1-1.
a b Janeway C, Travers P 1994. Immunobiology : The Immune System in Health and Disease. London; San Francisco; New York: Current Biology Limited; Garland Pub. Inc., ISBN 0-8153-1691-7. page needed
a b Janeway CA, Travers P, Walport M, Capra JD 1999. Immunobiology: The Immune System in Health and Disease 4th ed., 635p. New York: Garland Pub, ISBN 0-8153-3217-3. page needed
Janeway s 1999 4th edition. p. 341. full citation needed
a b c Abbas AK, Lichtman AH 2003. Cellular and Molecular Immunology 5th ed., 563p. Philadelphia: Saunders, ISBN 0-7216-0008-5. page needed
Peakman M, Vergani D 1997. Basic and Clinical Immunology. New York: Churchill Livingstone, ISBN 0-443-04672-7. page needed
Paul WE ed. 1999. Fundamental Immunology 4th ed., 1589p. Philadelphia: Lippincott-Raven, ISBN 0-7817-1412-5. page needed
Sims PJ, Wiedmer T 2000. Complement biology, In Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, eds. 2000. Hematology: Basic Principles and Practice, 3rd ed. pp. 651–667, New York; Edinburgh: Churchill-Livingstone, ISBN 0-443-07954-4.
Frank K, Atkinson JP 2001 Complement system. In Austen KF, Frank K, Atkinson JP, Cantor H. ed. Samter s Immunologic Diseases 6th ed. Vol. 1, p. 281–298, Philadelphia: Lippincott Williams Wilkins, ISBN 0-7817-2120-2.
a b c Roitt I, Brostoff J, Male D 2001. Immunology 6th ed., 480p. St. Louis: Mosby, ISBN 0-7234-3189-2. page needed
Anderson DM 2003 Dorland s Illustrated Medical Dictionary 30th ed., Philadelphia: W.B. Saunders, ISBN 0-7216-0146-4. page needed
Parham P 2005. The Immune System. New York: Garland, ISBN 0-8153-4093-1. page needed
a b c d e Murphy K, Travers P, Walport M, with contributions by Ehrenstein M et al. 2008. Janeway s Immunobiology 7th ed., New York: Garland Science, ISBN 0-8153-4123-7. page needed
Atkinson JP 2009. Complement system, In Firestein GS, Budd RC, Harris ED Jr, McInnes IB, Ruddy S, Sergent JS, eds. 2009. Kelley s Textbook of Rheumatology, pp. 323–336, Philadelphia, PA: Saunders/Elsevier, ISBN 978-1-4160-3285-4.
Janeway CA Jr., Travers P, Walport M, Shlomchik MJ 2001. The complement system and innate immunity. Immunobiology 5th ed.. Garland Publishing. ISBN 0-8153-3642-X.
Doan T, Melvold R, Viselli S, Waltenbaugh C 2007. Lippincott s Illustrated Reviews: Immunology, 320p. Lippincott Williams Wilkins page needed
DeFranco AL, Locksley RM, Robertson M 2007. Immunity : The Immune Response in Infectious and Inflammatory Disease. London; Sunderland, MA: New Science Press; Sinauer Associates, ISBN 978-0-9539181-0-2. page needed
Ammitzbøll, Christian Gytz; Kjær, Troels Rønn; Steffensen, Rudi; Stengaard-Pedersen, Kristian; Nielsen, Hans Jørgen; Thiel, Steffen; Bøgsted, Martin; Jensenius, Jens Christian 2012. Non-Synonymous Polymorphisms in the FCN1 Gene Determine Ligand-Binding Ability and Serum Levels of M-Ficolin. PLoS ONE 7 11 : e50585. Bibcode:2012PLoSO750585A. doi:10.1371/journal.pone.0050585. PMC 3509001. PMID 23209787.
Arumugam, Thiruma V; Shiels, Ian A; Woodruff, Trent M; Granger, D Neil; Taylor, Stephen M 2004. The Role of the Complement System in Ischemia-Reperfusion Injury. Shock 21 5 : 401–9. doi:10.1097/00024382-200405000-00002. PMID 15087815.
Naesens, M.; Li, L.; Ying, L.; Sansanwal, P.; Sigdel, T. K.; Hsieh, S.-C.; Kambham, N.; Lerut, E.; Salvatierra, O.; Butte, A. J.; Sarwal, M. M. 2009. Expression of Complement Components Differs Between Kidney Allografts from Living and Deceased Donors. Journal of the American Society of Nephrology 20 8 : 1839–51. doi:10.1681/ASN.2008111145. PMC 2723986. PMID 19443638.
Sacks, Steven H; Chowdhury, Paramit; Zhou, Wuding 2003. Role of the complement system in rejection. Current Opinion in Immunology 15 5 : 487–92. doi:10.1016/S0952-7915 03 00100-6. PMID 14499254.
Galvan, M. D.; Luchetti, S.; Burgos, A. M.; Nguyen, H. X.; Hooshmand, M. J.; Hamers, F. P. T.; Anderson, A. J. 2008. Deficiency in Complement C1q Improves Histological and Functional Locomotor Outcome after Spinal Cord Injury. Journal of Neuroscience 28 51 : 13876–88. doi:10.1523/JNEUROSCI.2823-08.2008. PMC 2680920. PMID 19091977.
Nguyen, Hal X; Galvan, Manuel D; Anderson, Aileen J 2008. Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal cord injury. Journal of Neuroinflammation 5: 26. doi:10.1186/1742-2094-5-26. PMC 2443364. PMID 18578885.
Beck, K. D.; Nguyen, H. X.; Galvan, M. D.; Salazar, D. L.; Woodruff, T. M.; Anderson, A. J. 2010. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133 2 : 433–47. doi:10.1093/brain/awp322. PMC 2858013. PMID 20085927.
Dragon-Durey, Marie-Agnès; Frémeaux-Bacchi, Véronique 2005. Atypical haemolytic uraemic syndrome and mutations in complement regulator genes. Springer Seminars in Immunopathology 27 3 : 359–74. doi:10.1007/s00281-005-0003-2. PMID 16189652.
Zipfel, Peter; Misselwitz, Joachim; Licht, Christoph; Skerka, Christine 2006. The Role of Defective Complement Control in Hemolytic Uremic Syndrome. Seminars in Thrombosis and Hemostasis 32 2 : 146–54. doi:10.1055/s-2006-939770. PMID 16575689.
Mooijaart, Simon P.; Koeijvoets, Kristel M.C.; Sijbrands, Eric J.G.; Daha, Mohamed R.; Westendorp, Rudi G.J. 2007. Complement Factor H polymorphism Y402H associates with inflammation, visual acuity, and cardiovascular mortality in the elderly population at large. Experimental Gerontology 42 11 : 1116–22. doi:10.1016/j.exger.2007.08.001. PMID 17869048.
Bradley, D T; Zipfel, P F; Hughes, A E 2011. Complement in age-related macular degeneration: A focus on function. Eye 25 6 : 683–93. doi:10.1038/eye.2011.37. PMC 3178140. PMID 21394116.
Datta, P.K.; Rappaport, J. 2006. HIV and complement: Hijacking an immune defense. Biomedicine Pharmacotherapy 60 9 : 561–8. doi:10.1016/j.biopha.2006.07.087. PMID 16978830.
Retrieved from https://en.wikipedia.org/w/index.php.title Complement_system oldid 697074573
Categories: Complement systemImmune system.
The complement system is a part of the immune system that enhances complements the ability of antibodies and phagocytic cells to clear pathogens from an organism.
IgM and IgG are the only immunoglobulin capable of activating complement classical pathway. Complement activation can be initiated by complex polysaccharides or.